Movement is part of life for most organisms. Movement is necessary for life to persist. The term migration is rich with social, economic, and political connotations these days, but for a life scientist, the act of migration is a requirement for ecological and evolutionary success. We recognize that most animals move, but tend to perceive plants as static, lacking the legs, fins, wings, slithering bellies, and cilia to change location. Of course, this is nonsense; plants move but in a different mode and tempo than their animal neighbors. Appreciating the universality of movement in the living world may put human migrations in a broader, more benign context.
The presence of humans on all continents is itself an outcome of past migrations throughout our evolutionary history. Setting off from the East African savannas, our species quickly moved through Old World habitats and then towards the end of the last glaciation about 13,000 years ago entered the New World. We spread quickly from Bering Sea beaches to Tierra del Fuego slopes, migration whizzes that we are. This global human migration caused enormous changes to the Americas’ landscape, as these first immigrant humans hunted and burned, adding their culture as a contributor to the ecological community structure [1]. Later immigrants and their descendants, now in the millions, continue to redesign the physical and living landscape.
Animal dispersal fruit. The plant supplies the reward, a fleshy high-caloric covering of the seeds, and the vertebrate animal provides the transport for seeds, released through the mouth or vent. Thousands of species, including we humans, participate in this mutualism. Photo by S. N. Handel.
Our botanical neighbors also move over time. During an annual cycle, most plants disperse seed and pollen through wind or by animal partners. The seed migration expands existing populations or starts new ones at some distance, sometimes hundreds of kilometers away from the mother plant. This long-distance dispersal secures species persistence even if the original population is destroyed by disturbance, whether by physical or biotic forces. Floods, fires, scouring by glaciers, and ecological succession all can overwhelm a plant population; dispersal (= migration) is necessary to avoid population extirpation or even extinction if the entire species range is overwhelmed.
Pollen dispersal also moves genes around, increasing the adaptive potential of the next generation. Pollen movement drives genotypic diversity upon which natural selection acts. Most plant species reproduce sexually, with pollen, migrating from near or far, fertilizing the mother plants’ ovules. New genotypes created by pollen movement respond to changing environmental conditions; some persist, and others that are not adaptive to the new conditions are bad genetic bets and are lost. Diversity borne of pollen movement builds resilience, enhancing a population’s ability to persist through hard times.
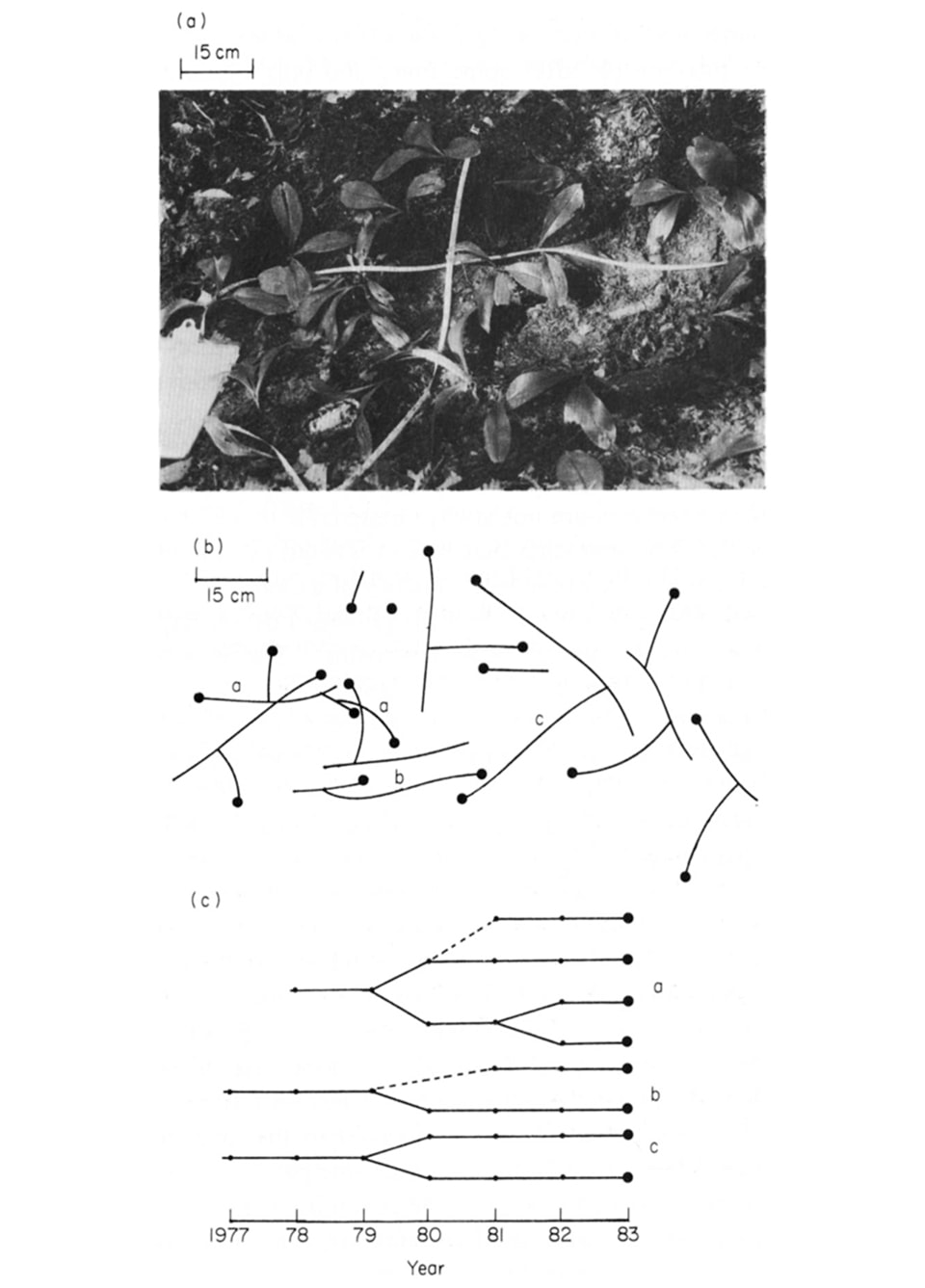
Plant migration across the forest floor. Clones, genetically identical parts of a plant from a single seedling, move away from the seedling initiation position over time. For example, for Clintonia borealis: (a) Appearance of a patch, showing scattered rosettes (b) Map of the same patch after removal of soil, showing the underground connections among rosettes. (c) Diagrams of branching rhizome fragments ‘a,’ ‘b,’ and ‘c’ from the previous picture, showing the annual nodes. Note for ‘a’ and ‘b’ that dormant rhizome buds produced in 1980 and 1979, respectively, renewed growth in 1981 [2].
Change in the environment can be observed at the scale of the microhabitat or an entire continent. Plant movements are known across this entire spatial range. One example of small-scale movement is clonal growth, in which a single plant expands by rhizome, runner, or fragmentation to spread a single genotype across nearby spaces. In a typical example, the woodland wildflower Clintonia borealis’ underground rhizomes grow laterally from a shoot a few centimeters each year. The next year the original shoot dies, and new shoots emerge further from the initial point of seed germination. Over time, a single plant genotype can move many centimeters, exploring new pieces of the forest floor where nutrients and water resources may be more benign [3].
Hundreds of species such as clovers, strawberries, milkweeds, aspens, or sumacs advance clonally in similar fashion. Over years some clones can become enormous, covering hundreds of square meters. In addition to incremental growth, other species, such as Salix (willow), make use of an even more dynamic process, such as fragmentation, in which bits of shoots are carried away by water currents. These bits of plant material can bang into a muddy bank and start a new plant clonally identical to the source individual some distance from the mother plant. Falling apart is a creative action for these species, not an organic meltdown.
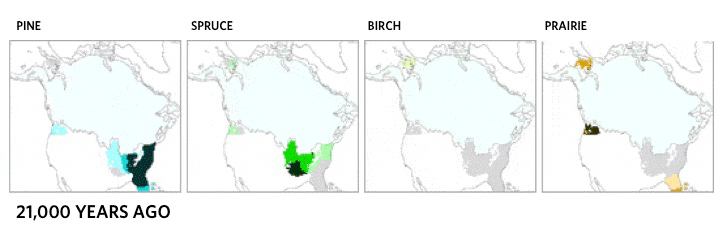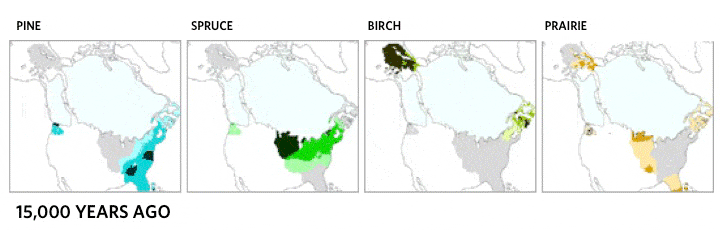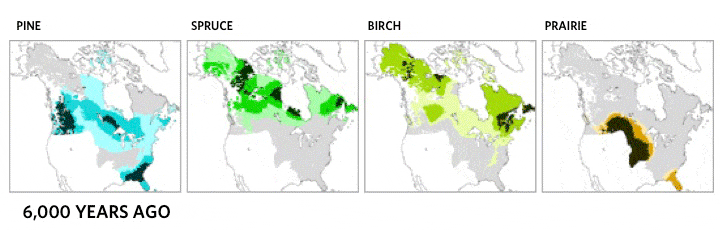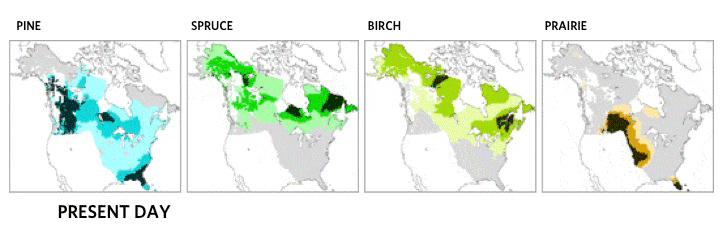
This very local scale of clones spreading may be critical ecologically, but it does not match the drama of continental plant movements, which occur over longer time spans. Records of the northern movement of plant species as the glaciers melted during the Holocene show the steady march of common canopy tree species across hundreds of kilometers [4]. Each species moved at its own distinct and idiosyncratic tempo, reaching new lands and often leaving behind former landscapes. Consequently, forest community structure and membership shifted over time as the various species migrated.
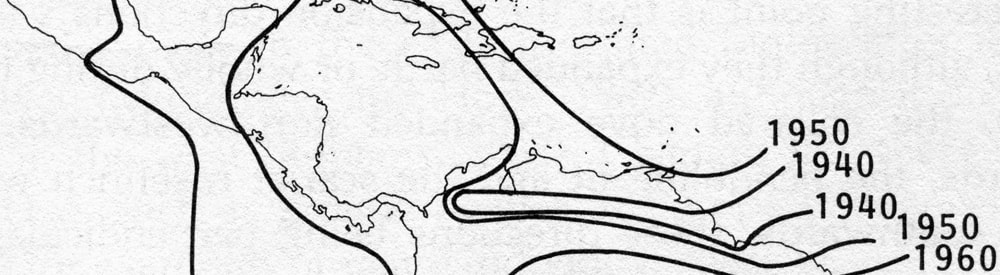
Migration of North American boreal tree species since the last ice age, from 21,000 years ago to the present day. Based on images by the NASA Earth Observatory, the Department of Geological Sciences at Brown University, the National Center for Ecological Analysis and Synthesis, and the Department of Geography at the University of Oregon [5].
The migration rates of individual species and populations are set by inherent ecological factors such as seed and fruit size, age at maturation, and identity and prevalence of the migration agent (animals, water, or wind). Physical barriers to migration slow species spread across the landscape. Water bodies, stretches of unfavorable soils, and mountain ranges (with inappropriate conditions at higher elevations) all can all act as impediments that prevent species from reaching even climatically appropriate zones. Biotic barriers also slow geographic spread. Predators and pests present in a new area can be as effective in stopping migration as a wall or an ocean.
With rapid climate change now affecting all terrestrial and aquatic habitats, patterns of migrations will again be altered [6]. Different species will have idiosyncratic responses to the new climate regimes, and natural communities will have changing memberships [7]. Human-dominated areas form major hurdles for plant and animal migrations, as the large areas of hardscape and lands managed for our own needs (agriculture, sports, amenity) challenge indigenous species to continue their movements. The stresses of human activities further reduce survivorship across many age classes of plants and animals. Mowing, for example, effectively sterilizes many plants before flowering structures are even formed. The developed world is a maze with many dead ends and cul-de-sacs for species on the move.
Our increased urbanization traps species from moving as climate envelopes shift. There is interest in proactively moving species to keep pace with climate change [8]. This is an enormous undertaking, whose success rests on the accurate prediction of future environmental conditions, but the crystal ball is cloudy. Little precision to match species with future microclimates is available. Assisted migration, the practice of proactively moving species northward or up in elevation to find the appropriate climatic regime, is being attempted on a modest scale, most often for rare species where static distributions may preface extinction [9].
Although this approach has resonated with members of the conservation community, there are many ecological and social barriers to the success of this extreme gardening approach [10]. Single organisms often rely on others, mutualists, for reproductive success, and the entire suite of associated species is difficult to move along with the focal species. Relocating a new species into an existing community may also have unintended deleterious effects. Presently, there is neither financial nor governmental support to move hundreds of species to counter the challenge of climate change. Assisted migration is a concept that simply may not be scalable to predicted ecosystem needs.
Plant reintroduction is a related action, attempted after a species has been lost to some unfavorable conditions and a restoration of the species into its past range is attempted. This would only be effective if the causative stress has been removed or actions to stop a reoccurrence can be assured. Reintroduction is not a migration action, but a return to a historic biotic structure.
New research shows that the velocity of climate change is not even, but varies with local topographies [11]. This adds another factor that will determine the composition of future natural communities, increasing the discordance of the future from the past. When human populations move, these migrations change the character of societies in many ways. In the natural world, an analogous dynamic occurs and has always occurred: the past in not a predictor of the future. Natural and human communities are ever-changing.
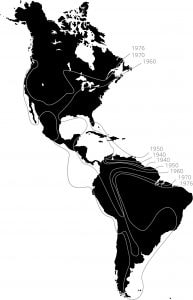
Range expansion of the Cattle Egret. This species (Bubulcus ibis) was first recorded in South America in the1870’s after a natural dispersal, probably by storms, from its native Africa. It remained local until the 1930’s, then spread through most of the hemisphere within the next forty years [12]. This pattern is typical of many cases of rapid range expansion of species after episodic long-distance dispersal. Image based on Hengeveld, 1989
The other major plant and animal migration process that is shaping our biotic world is the introduction of new species from distant continents. Many species from Asia, Europe, and Africa have become abundant members of our western hemisphere biota, and other taxa have made the reverse migration from the Americas to the Eastern Hemisphere. In their new geographical context, many of these species have extremely high growth rates. Often their new landscapes lack former natural enemies that would have evolved to exploit them as food sources. This raises the carrying capacity of the migrants in their new range. This relationship has been proven many times by investigators discovering and releasing the former predators in the new range and then monitoring the retreat of the invasive plants. The introduction of opuntia moths in Australia and purple loosestrife beetles in North America provides convincing and well-documented examples of this management practice.
The speed of the intercontinental migrations is now extremely rapid, as people and cargo move across oceans at rapid rates. High-speed jet and ship traffic allow species to stay alive until landfall; in past decades and centuries the long transoceanic passages took weeks or months, and many of the ecological stowaways died en route. Consequently, plant and animal migrations are occurring at faster rates. Occasionally an enemy species is also found and the subsequent its migration limits the damage of the earlier invader. Context and effect must be understood before species migrations are branded as problems; sometimes they are ecological cures.
Invasive species migration has had a major impact on the composition and sustainability of historic species interrelationships [13]. Often when non-native species arrive in a landscape, biodiversity and abundance of natives fall and food webs are diminished. For example, non-native plant species may not support insect guilds that relied on the now less common native species. Predators of these insects such as birds and parasitoids, in turn, are denied resources as insect populations shrink along with their now diminished plant food source [14].
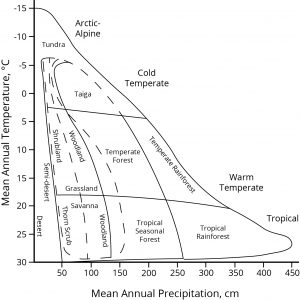
The major present-day biome types of the world, arrayed by temperate and precipitation. Climate change is expressed along both axes. Global distribution of these biome types will inevitably move in position and extent on this graphic in still imprecisely-known trajectories. Based on Whittaker, 1975, p. 167.
In our human-dominated geological era, the Anthropocene, the movements of species and the restructuring of ecological communities has been described as creating a world of novel ecosystems [15]. However, the known processes of short- and long-scale species migration suggest that the living world is always “becoming” novel in some way and that continuing change in local species membership is the normal ecological condition. We must not confuse the past with the future, knowing that the past was not static but has always experienced change. Attempting to stop species migrations and habitat transformation belies processes that have been continually expressed for millennia.
In charting natural habitat types across the world, the major biome types can be diagrammed along two axes — of temperature and of precipitation. Climate change is occurring along both these axes, and as the positions of the biome types inevitably move, the relative proportions of land that the biomes cover must also change. Future habitat reconfiguration across the Earth’s surface will be the largest scale change people will experience, the grandest of natural migrations. Buckle up; it will probably be a fast and mysterious ride.
 Steven Handel, Distinguished Professor of Ecology and Evolution at Rutgers University and Visiting Professor of Ecology at Harvard University Graduate School of Design, studies the restoration ecology of urban and degraded habitats and how this can mesh with landscape architecture design. He serves as Editor of the journal Ecological Restoration and is an Aldo Leopold Leadership Fellow of the Ecological Society of America. He received the Theodore Sperry Award in 2011 from the Society for Ecological Restoration, for his work on urban habitat creation, and is an Honorary Member of the American Society of Landscape Architects. He received his B.A. in biology from Columbia College and PhD in ecology and evolution from Cornell University and also has taught at Yale and Stockholm Universities.
Steven Handel, Distinguished Professor of Ecology and Evolution at Rutgers University and Visiting Professor of Ecology at Harvard University Graduate School of Design, studies the restoration ecology of urban and degraded habitats and how this can mesh with landscape architecture design. He serves as Editor of the journal Ecological Restoration and is an Aldo Leopold Leadership Fellow of the Ecological Society of America. He received the Theodore Sperry Award in 2011 from the Society for Ecological Restoration, for his work on urban habitat creation, and is an Honorary Member of the American Society of Landscape Architects. He received his B.A. in biology from Columbia College and PhD in ecology and evolution from Cornell University and also has taught at Yale and Stockholm Universities.
Notes
[1] Shepard Krech, The Ecological Indian: Myth and History (New York: WW Norton & Company, 2000); Paul Schultz Martin, Twilight of the Mammoths: Ice Age Extinctions and the Rewilding of America, (Berkeley: University of California Press, 2005); Elizabeth Kolbert, The Sixth Extinction: An Unnatural History (New York: Henry Holt and Company, 2014).
[2] Mark W. Angevine and Steven N. Handel, “Invasion of forest floor space, clonal architecture, and population growth in the perennial herb Clintonia borealis,” The Journal of Ecology (1986): 547-560.
[3] Angevine and Handel, 1986.
[4] Margaret B. Davis, “Quaternary history of deciduous forests of eastern North America and Europe,” Annals of the Missouri Botanical Garden (1983): 550-563.
[5] Rebecca Lindsay, “The Migrating Boreal Forest,” NASA Earth Observatory, August 20, 2002, https://earthobservatory.nasa.gov/Features/BorealMigration/printall.php.
[6] Margaret B. Davis and C Zabinski, “Changes in geographical range resulting from greenhouse warming: effects on biodiversity in forests,” Global warming and biological diversity (1992): 297-308.
[7] Louis R. Iverson, Anantha M. Prasad, Stephen N. Matthews, and Matthew Peters, “Estimating potential habitat for 134 eastern US tree species under six climate scenarios,” Forest Ecology and Management 254(3) (2008): 390-406.
[8] Joyce Maschinski and Kristin E. Haskins, Plant Reintroduction in a Changing Climate: Promises and Perils (Washington, D.C.: Island Press, 2012).
[9] Pati Vitt, Kayri Havens, Andrea T. Kramer, David Sollenberger, and Emily Yates, “Assisted Migration of Plants: Changes in Latitudes, Changes in Attitudes,” Biological Conservation 143(1) (2010): 18-27.
[10] Jason S. McLachlan, Jessica J. Hellmann, and Mark W. Schwartz, “A Framework for Debate of Assisted Migration in an Era of Climate Change,” Conservation Biology 21(2) (2007): 297-302.
[11] Scott R. Loarie, Philip B. Duffy, Healy Hamilton, Gregory P. Asner, Christopher B. Field, and David D. Ackerly, “The Velocity of Climate Change,” Nature 462(7276) (2009): 1052-1055.
[12] Gilbert T. Crosby, “Spread of the Cattle Egret in the Western Hemisphere,” Bird-Banding 43 (1972): 205-212.
[13] Don C. Schmitz, Daniel Simberloff, Ronald H. Hofstetter, William Haller, and David Sutton, “The Ecological Impact of Nonindigenous Plants,” in Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida ed. Daniel Simberloff, Donald C. Schmitz, and Tom C. Brown, (Washington D.C.: Island Press, 1997): 39-61.
[14] Karin T. Burghardt, Douglas W. Tallamy, and W. Gregory Shriver, “Impact of native plants on bird and butterfly biodiversity in suburban landscapes,” Conservation Biology 23 (1) (2009): 219-224.
[15] Richard J. Hobbs, Eric S. Higgs, and Carol Hall, Novel ecosystems: intervening in the new ecological world order (Chichester: John Wiley & Sons, 2013); Carolina Murcia, James Aronson, Gustavo H. Kattan, David Moreno-Mateos, Kingsley Dixon, and Daniel Simberloff, “A Critique of the ‘Novel Ecosystem’ Concept,” Trends in Ecology & Evolution 29(10) (2014): 548-553.